The Problem:
There is currently no way to quickly detect inflammation, leading to invasive surgeries
There is currently no way to quickly detect inflammation, leading to invasive surgeries
A temperature sensor that warns of disease flareups and tracks disease progression in real time.
The approach offers long-term, real-time monitoring and could enable clinicians to act earlier to prevent or limit the permanent damage caused by inflammatory episodes.
Arun Sharma, Research Associate Professor of Urology, Research Associate Professor of Biomedical Engineering; John Rogers, Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering, and Neurological Surgery
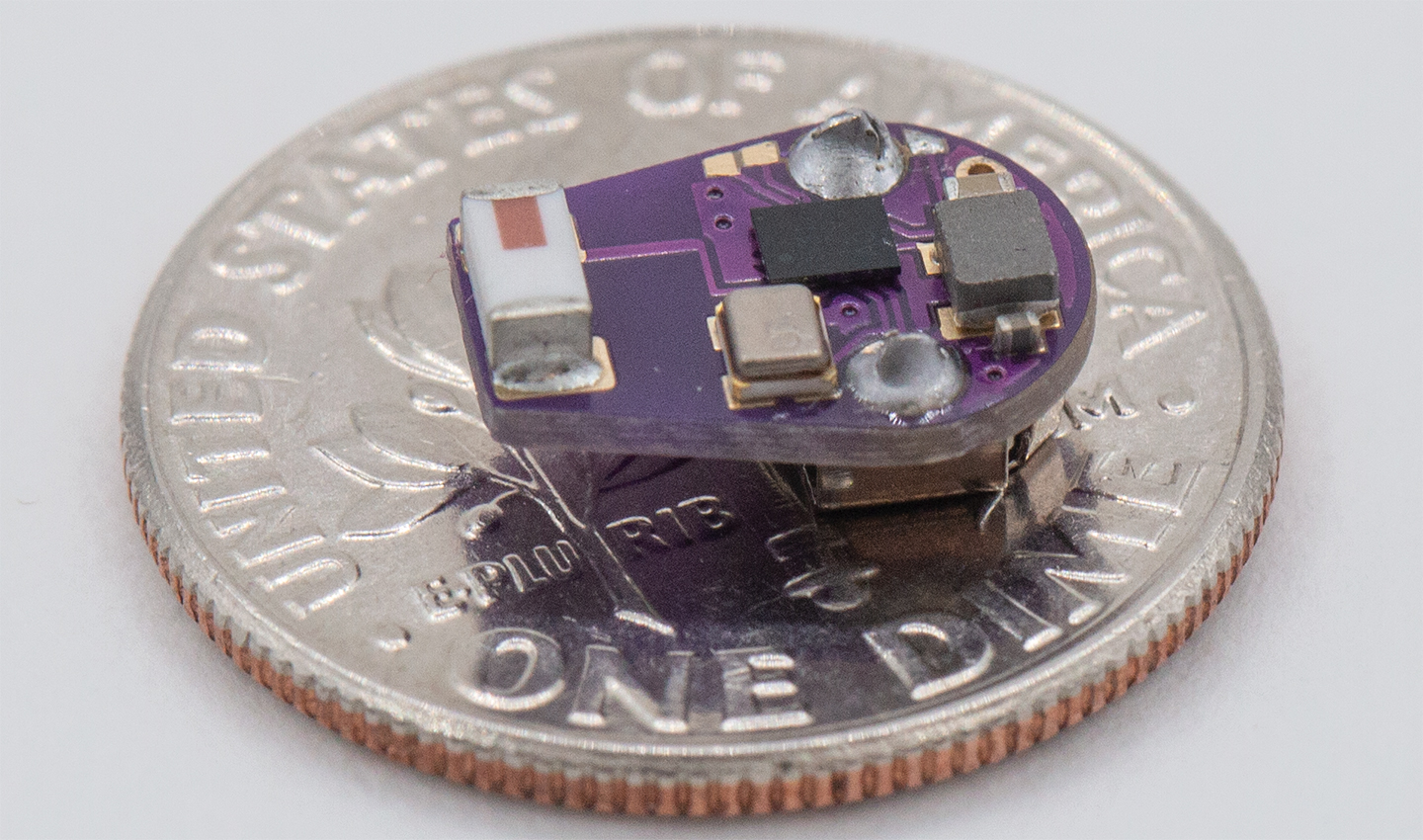
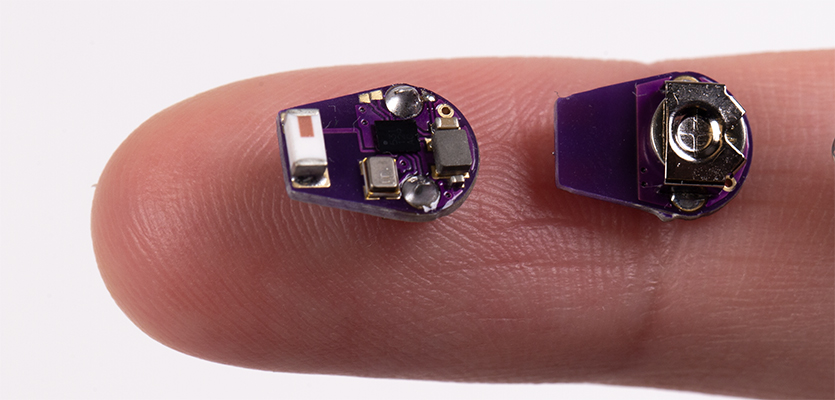
A team of Northwestern University scientists has developed the first wireless, implantable temperature sensor to detect inflammatory flareups in patients with Crohn’s disease. The approach offers long-term, real-time monitoring and could enable clinicians to act earlier to prevent or limit the permanent damage caused by inflammatory episodes.

More than 1 million Americans have Crohn’s disease, a chronic inflammatory bowel disease that affects the intestines, causes digestive issues, and can lead to weight loss, malnutrition, and other complications. People with mild cases are treated with oral medications, but these drugs typically fail over time, requiring approximately 70 percent of Crohn’s patients to undergo at least one surgery to remove portions of damaged intestines.
Because heat is indicative of inflammation, the Northwestern scientists tested whether a temperature sensor resting gently against the intestines of mice with Crohn’s disease could provide real-time insight into the progression of the disease, as well as detect episodic flareups. Sure enough, they accomplished both goals in their research, was published online March 18 in the journal Nature Biomedical Engineering.
Arun Sharma, whose group led the animal testing, said there is currently no way for clinicians to quickly detect inflammatory events, some of which go unnoticed by patients until the problem becomes so severe that it requires invasive surgery.
“The magnitude of the flareup can be measured with regards to the heat signature,” said Sharma, co-corresponding author on the paper and a research associate professor of urology at Northwestern University Feinberg School of Medicine and of biomedical engineering at Northwestern Engineering. “Is it so extensive that it’s going to cause tissue damage over time?
“This could be potentially prevented if a clinician has this information readily at hand and can determine what type of therapy can be given to that person at that moment in time, rather than waiting weeks to get a blood analysis, tissue biopsy, or fecal analysis. In the meantime, you’re losing valuable minutes regarding tissue damage with this inflammatory event.”
Sharma said this strategy of measuring temperature fluctuations could also be useful for patients with ulcerative colitis, another inflammatory bowel disease, or any condition where there is a prolonged inflammatory response. In their study, the researchers used the wireless sensors to continuously track temperature fluctuations for nearly four months.
Bioelectronics pioneer John Rogers, whose group led the device development, recently published another paper describing an ultrathin, soft implant that measures temperature and perfusion changes as a way to monitor the health of transplanted organs. Once again, the relationship between heat and inflammation was key, as excess inflammation around the transplanted organ can offer an early warning sign that the new organ is being rejected by the patient’s immune system.
“To address Crohn’s disease, we developed an ultraminiaturized, precision temperature sensor with wireless communication capability,” said Rogers, co-corresponding author on the paper. “This tiny, soft device takes the form of a smooth, round capsule that rests within the GI system, without affecting natural physiological processes for long-term recordings. The data show some very unique signatures, in the form of perturbations to natural circadian cycles, known as ultradian rhythms, as early indications of inflammatory responses.”
The scientists discovered that the ultradian temperature rhythms correlate to cyclic variations in stress levels and inflammatory markers in blood, said Surabhi Madhvapathy, co-first author from Rogers’ group who led the sensor engineering.
“In addition to the short-term variations, we learned over the span of weeks to months, that the average temperature of the intestines decreases,” Madhvapathy said. “This decrease was indicative of the worsening tissue quality over time.”
Following these successful results in mice, the researchers plan to assess the sensor capabilities in human tissues that recreate the inflammatory gut conditions found in Inflammatory Bowel Disease.
Rogers is the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering, and Neurological Surgery at the McCormick School of Engineering, and director of the Querrey Simpson Institute for Bioelectronics, which supported the study. Sharma is director of Pediatric Urological Regenerative Medicine and Surgical Research at Ann & Robert H. Lurie Children’s Hospital of Chicago and the Stanley Manne Children’s Research Institute, and a member of Northwestern’s Simpson Querrey Institute for BioNanotechnology.
Madhvapathy of the Rogers group and Matthew Bury of the Sharma group are co-first authors of the paper.