The Problem
Iridium-based catalysts are widely used in green energy technologies, but existing methods to measure active sites on iridium catalysts are imprecise.
Iridium-based catalysts are widely used in green energy technologies, but existing methods to measure active sites on iridium catalysts are imprecise.
A technique called mercury underpotential deposition to create a temporary, single mercury layer on iridium catalysts.
The advancement could impact how researchers study iridium-based catalysts, potentially leading to tangible outcomes for improving sustainable technologies.
Assistant Professor Linsey Seitz
New research from Northwestern Engineering’s Linsey Seitz provides new insight on how to measure active parts of catalysts, an essential step toward improving materials used in clean energy technologies.
The advancement could impact how researchers study iridium-based catalysts, widely used in several green energy applications, including hydrogen production through proton exchange membrane water electrolysis (PEMWE).
“This work could enable more reproducible, informed, and meaningful research regarding fundamental electrocatalyst activity evaluation that can be more effectively translated to tangible outcomes for improving sustainable technologies,” Seitz said.
Seitz is an assistant professor of chemical and biological engineering at the McCormick School of Engineering. A faculty affiliate of the Paula M. Trienens Institute for Sustainability and Energy, Seitz reported her research in “Quantification of Electrochemically Accessible Iridium Oxide Surface Area with Mercury Underpotential Deposition,” published November 6 in Science Advances.
A critical piece of developing sustainable technology for cleaner energy and fuel production lies in creating catalyst materials that can reliably and efficiently power key chemical processes. Scientists are tasked with developing catalysts that not only drive specific reactions but also remain stable over time and through demanding conditions. But even getting an accurate read on these performance goals is a challenge of its own.
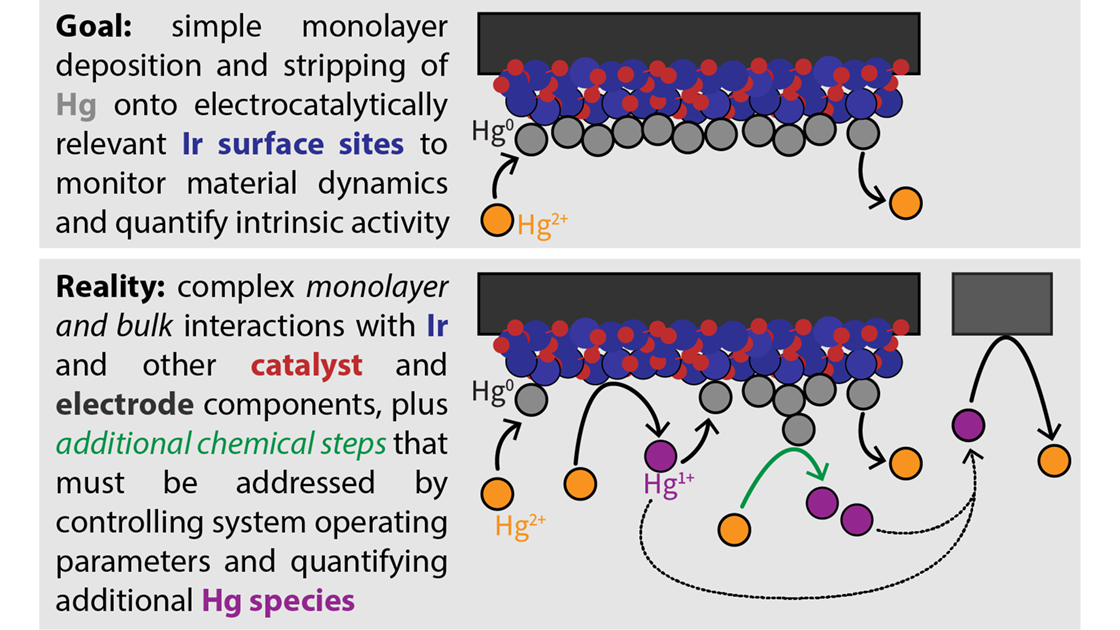
Seitz developed a method that uses mercury underpotential deposition (UPD) — a reaction where mercury creates a temporary single layer on the iridium. Researchers can then carefully strip away the mercury to determine which iridium sites are actively contributing to the reaction, gaining a more accurate sense of active iridium sites. Previous attempts at using mercury UPD have struggled with inconsistent analyses and measurements. Seitz’s team, however, used advanced electrochemical and materials analysis to isolate the specific reactions happening on the iridium surface, making it possible to measure only the sites relevant to the catalytic process.
Currently, the best candidate for PEMWE technologies is iridium, a rare and costly metal. To make green hydrogen production economically viable, researchers are working to find ways to use as little iridium as possible while maximizing the effectiveness of each atom in the reaction. But accurately measuring how many iridium atoms are actively involved in the catalytic process has been difficult due to limited tools and methods. Current techniques only offer rough estimates, which often include non-active materials or don’t account for changes that happen during use.
Seitz’s study could represent a step forward.
“The mercury UPD protocol we develop and propose within this manuscript enables first-of-its-kind, transformative intrinsic activity characterization for electrochemical performance evaluation across a wide variety of iridium-based electrocatalyst materials,” Seitz said. “We published this paper open-access as we provide a novel protocol recommendation that varies significantly from prior work in this space to provide future researchers with a quantification and activity normalization method that is inherently robust and based in the proof of its underlying physical phenomena and chemistry.”
Seitz and her teammates aimed to provide new information on mercury-based electrochemistry, identifying mechanisms for mercury adsorption and stripping on individual and combined components that comprise the complex catalyst/electrode structures employed in PEMWE technologies.
“This work is a particularly impactful characterization of iridium-based catalyst materials, even highly complex structures, which are ubiquitously used for green hydrogen production via PEMWE, as well as other processes,” Seitz said.
Moving forward, Seitz plans on applying this technique to a wider range of catalyst materials to bring new levels of insights to the actual intrinsic activities, and provide a new benchmarking metric for critical analysis of the collective progress toward enhancing electrocatalytic efficiency for a wide range of emerging sustainable technologies.
“We envision this work enabling new catalyst design principles as more accurate, robust, and relevant assessments of catalyst activities will now be possible to catalog and analyze in parallel with material properties that support enhanced performance,” Seitz said.